Measures used in clinical trials of depression only partially reflects what matter to patients
Research waste occurs when randomized controlled trial (RCT) outcomes are heterogeneous, or overlook domains that matter to patients (e.g., those relating to symptoms or functions).
In this methodological systematic review published in The Lancet Psychiatry, Christopher Veal and colleagues (CRESS’s METHODS Team) evaluated the heterogeneity of outcomes measured in depression trials and their relevance to patients. Veal and colleagues extracted all outcome measures used in RCTs of adult unipolar and bipolar depression registered between 2018 and 2022. Then, patients and clinicians matched each item within the 25 most frequently used measures with the 80 domains of depression that matter to patients previously identified by Astrid Chevance et al.
Among the 450 trials, Veal et al. identified 388 different measures, with a maximum of 40% of the RCTs using the same measure (Hamilton Depression Rating Scale [HAMD]). Seven (9%) domains were not covered by the 25 most frequently used outcome measures (e.g., mental pain, emotional regulation, mood reactivity irritability). The HAMD covered a maximum of 47 (59%) of the 80 domains that matter to patients.
Veal and colleagues underscore the need to address outcome measure heterogeneity in RCTs, which is crucial for facilitating effective comparison and synthesis of trial results. Whilst efforts to standardise outcomes and their measures in depression research are ongoing, including the development of a Core Outcome Set (COS) for depression by Astrid Chevance and colleagues, the review’s findings also signal a need for caution in standardising outcomes, as complementary measures may need to be included alongside commonly used measures to ensure comprehensive coverage of patient-relevant domains. The review’s findings emphasise the critical need for greater alignment between patients’ experiences and the outcome measures used for depression research.
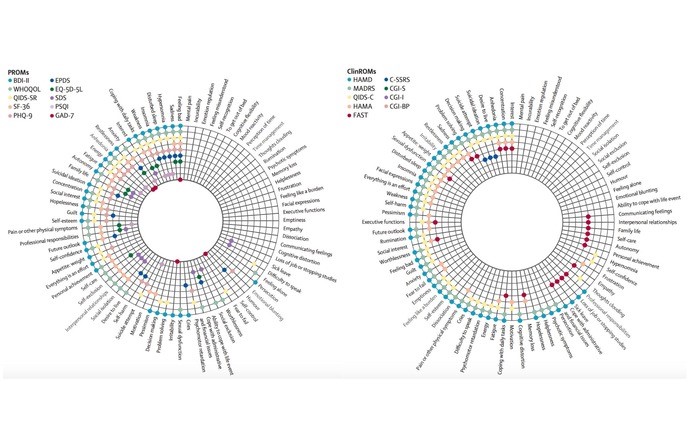
Photo credit : Veal, C., Tomlinson, A., Cipriani, A., Bulteau, S., Henry, C., Müh, C., … & Chevance, A. (2024). Heterogeneity of outcome measures in depression trials and the relevance of the content of outcome measures to patients: a systematic review. The Lancet Psychiatry, 11(4), 285-294.
Christopher Veal completed his MSc in Public Health in Comparative Effectiveness Research (Université Paris Cité) in 2022 which served as the foundation for this publication. He is passionate about patient-centred research and is currently developing a doctoral project to develop a novel methodology for assessing the content of subjective outcome measures
By Christopher Veal – christopher-james.veal@inserm.fr
- Veal C, Tomlinson A, Cipriani A, Bulteau S, Henry C, Müh C, Touboul S, De Waal N, Levy-Soussan H, Furukawa TA, Fried EI, Tran VT, Chevance A. Heterogeneity of outcome measures in depression trials and the relevance of the content of outcome measures to patients: a systematic review. Lancet Psychiatry. 2024 Apr;11(4):285-294. doi: 10.1016/S2215-0366(23)00438-8. PMID: 38490761.
- Chevance A, Ravaud P, Tomlinson A, Le Berre C, Teufer B, Touboul S, Fried EI, Gartlehner G, Cipriani A, Tran VT. Identifying outcomes for depression that matter to patients, informal caregivers, and health-care professionals: qualitative content analysis of a large international online survey. Lancet Psychiatry. 2020 Aug;7(8):692-702. doi: 10.1016/S2215-0366(20)30191-7. Erratum in: Lancet Psychiatry. 2020 Sep;7(9):e59. PMID: 32711710.
https://eiko-fried.com/what-depression-measure-is-best/ (Blog Post by co-author)