Background
This national study (Epipage 2) of preterm birth in France began 2011, conducted by the research team Obstetric, Perinatal and Pediatric Epidemiology (EPOPé), in collaboration with numerous regional teams of obstetricians, midwives, pediatricians, and epidemiologists.
Objectives
- To learn more about the outcomes of very preterm and late preterm babies
- To improve knowledge about the causes and consequences of preterm births
- To assess the effects of the organization of care and of medical practices on the health and development of preterm babies
- To improve our knowledge of the experiences of families and of the decision-making processes at childbirth and in the intensive care units
- To define specific healthcare and other services required during childhood.
- To study, in a global and multi-disciplinary manner, the major issues affecting the health, development and socialization of these children over the medium and long term.
Study methodology
Population
Epipage 2 is a cohort study of very preterm (22+0 to 31+6 weeks) and moderately preterm (32+0–34+6 weeks) babies, born in 21 regions of metropolitan France and 4 overseas districts (Martinique, Réunion, Guadeloupe, and French Guyana). The children were included at birth in 2011 (28 March–31 December 2011). Recruitment took place at birth in all maternity units in the participating regions.
Data collection
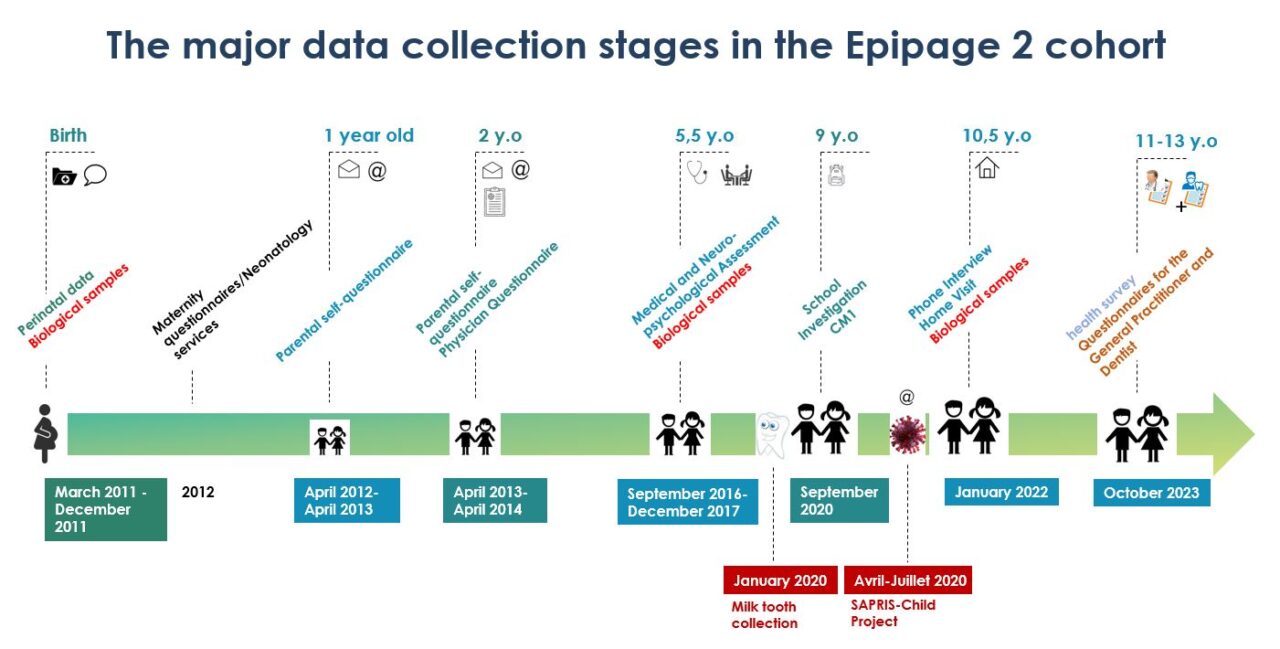
At birth, information about pregnancy, delivery, and immediate care of the child was collected in the maternity ward, from the medical files. At the end of the hospitalization in the neonatology department, a complete description of the care provided and medical complications was collected from the medical file. Information was also collected by an interview with each family and a self-administered questionnaire completed by the mothers.
Data were collected at several stages since the participants’ birth. Questionnaires were completed by families at 1, 2, 5 and a half years for the SAPRIS survey, and at 10 and a half years. Health professionals also completed questionnaires at 2 and 5 and a half years. The next stage of data collection will take place between the ages of 11 and 13, when data will be collected from doctors and dentists.
Follow-up of the children
The first stage of data collection, at a corrected age of 1 year, took place in 2012-2013. Parents were asked to complete a questionnaire about the child’s health and medical care during the first year, as well as the family’s health and living conditions.
The data collection at a corrected age of 2 years took place in 2013-2014 and included a questionnaire for the parents and another for the doctor, to assess as well as possible the children’s first stages of psychomotor development.
For the subsequent stages, assessments including medical examinations and psychological tests will be organized in regional centers at the ages of 5, 8, and 12 years.
Regional groups, supervised by the national coordination team, are responsible for the cohort follow-up.
Complementary projects
Projects complementary to EPIPAGE 2 are being conducted among some subgroups of children in some regions and/or some centers. The objective of these studies is to relate aspects of the children’s outcomes to: neonatal cerebral abnormalities on MRI (Epirmex), quantity and type of milk received during the first weeks of life (Epinutri), and intestinal flora (Epiflore). Others will study placental histology (Chorist), or possible genetic modifications observed in samples of cord and maternal blood (Biopag). Still others are looking at mother-child interactions (Olimpe) or the role of health-care professionals and parental involvement in decisions and practices related to palliative care in extremely preterm births (Ethics). Others focus on specific aspects of cognitive and language development (Epilang), early cognitive intervention (Epiremed), metabolism and neurocognitive development (Epivarec), or genetics and disease (Epipreterm). Other projects have also been initiated, such as the milk teeth collection phase, the CM1 school survey, and the SAPRIS survey, which focused on the Coronavirus epidemic (COVID-19).
Projects associated with EPIPAGE 2
National dimension
The Epipage 2 study is one of two cohorts making up the RE-CO-NAI platform (Plateforme de REcherche sur les COhortes d’enfants suivis depuis la NAIssance). As part of this platform, it is associated with the general Elfe cohort (18,300 children), implemented by a joint Ined-Inserm-Etablissement Français du Sang unit under the responsibility of Ined. The aim of the RE-CO-NAI project is to carry out a comprehensive, multidisciplinary study of the major issues surrounding children’s health, development and socialization (see more). Within the framework of this platform, all the follow-up steps implemented since 2020 are fully shared between the 2 cohorts.
European dimension
EPIPAGE 2 is also associated with the EPICE/ SHIPS European project, coordinated by Jennifer Zeitlin. Together they offer the opportunity to study the diversity of practices for the management of preterm children in Europe, an essential domain for understanding differences in results between countries and identifying effective therapeutic and preventive strategies (see more).
EPIPAGE 2 is also associated with the RECAP-Preterm project, coordinated by Jennifer Zeitlin. The aim of this project is to improve the health, development and quality of life of children and adults born very prematurely. It is based on the creation of a platform for gathering, harmonizing and exploiting data from 23 European cohort studies of very prematurely born children and adults from 13 European countries (see more).
Funding
- The Very Large Research Infrastructure program, through support from the French Institute of Public Health Research/Institute of Public Health and its partners: the French Health Ministry, the National Institute of Health and Medical Research (INSERM), the National Institute of Cancer, and the National Solidarity Fund for Autonomy (CNSA).
- The National Research Agency through the French EQUIPEX program for investments in the future (reference ANR-11-EQPX-0038 and ANR-19-COHO-001)
- The PREMUP Foundation
- Fondation de France (Reference 11779)
- Fondation pour la Recherche Médicale (SPF20160936356)
- Programme Hospitalier de Recherche Clinique Epinutri (DGOS13-040)
- Ministère de l’Enseignement Supérieur, De La Recherche et de L’Innovation (G13129KK)
- Apicil Foundation (R20065KK)
Partners
- Collège National des Gynécologues et Obstétriciens Français (CNGOF, French College of Gynecologists and Obstetricians)
- Société Française de Médecine Périnatale (SFMP, French Society for Perinatal Medicine)
- Société Française de Néonatologie (SFN, French Society of Neonatology)
- SOS Préma